Scientists Develop Advanced Catalyst for Self-driven Seawater Splitting with Enhanced Chloride Resistance
Seawater electrolysis has long been seen as a promising pathway for sustainable hydrogen production but has faced significant limitations due to chloride ion (Cl⁻) corrosion, which can degrade a catalyst’s performance.
Now, scientists from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences, along with their collaborators, have developed an efficient electrocatalyst called Co-N/S-HCS that demonstrates remarkable activity and stability in seawater electrolysis. This offers a sustainable hydrogen production solution with minimal reliance on freshwater resources.
"Our work has significantly enhanced the catalyst’s resistance to Cl⁻ corrosion by carefully tuning the electronic environment around cobalt atoms," said Dr. ZHANG Canhui, first author of the study and a researcher at QIBEBT. "This gives the Co-N/S-HCS both long-term stability and high activity."
Results of the study were published in Chem Catalysis on Nov. 13.
The Co-N/S-HCS electrocatalyst utilizes an asymmetric CoN₃S₁ structure, in which each cobalt (Co) atom is coordinated with three nitrogen (N) atoms and one sulfur (S) atom. This asymmetric CoN₃S₁ configuration, optimized through density functional theory and molecular dynamics simulations, modifies the electronic distribution around the Co center compared with the symmetric CoN4 configuration, thereby weakening corrosive Cl⁻ adsorption and enhancing the catalyst’s performance in seawater-based electrolytes.
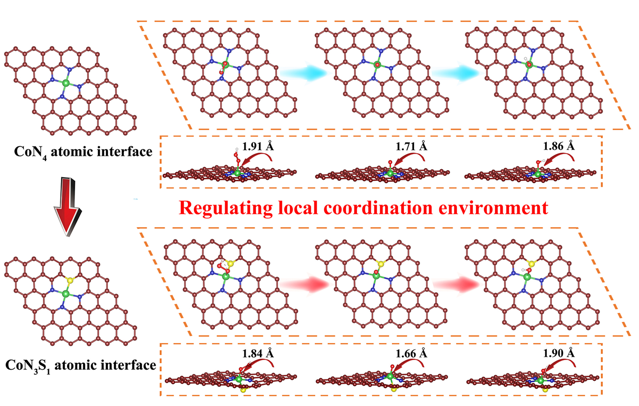
The DFT-optimized atomic configurations of oxygen intermediates (OOH*, O*, and OH*) adsorbed on symmetric CoN4 and asymmetric CoN3S1 atomic interface models (Image by ZHANG Canhui)
The tailored CoN₃S₁ configuration not only mitigates the corrosive effects of Cl- but also optimizes the catalyst’s trifunctional activity by enhancing its ability to facilitate key reactions such as oxygen reduction, oxygen evolution, and hydrogen evolution.
“This multipurpose capability is essential for practical applications in seawater-based energy systems,” said Dr. WANG Xingkun from QIBEBT, one of the study’s corresponding authors.
Researchers further validated their design by integrating Co-N/S-HCS into a self-driven seawater-splitting system. This system, coupled with seawater-based Zn-air batteries (S-ZABs) and two-electrode electrolysis devices, demonstrated impressive performance. The S-ZABs exhibited cycling stability for up to 650 hours, and the two-electrode electrolysis devices showed stable performance for over 1,100 hours.
More importantly, the integrated system has outperformed the CoSA/N, S-HCS system by achieving a higher hydrogen production rate of 469 µmol/h. Current methods of hydrogen production rate is 184 µmol/h.
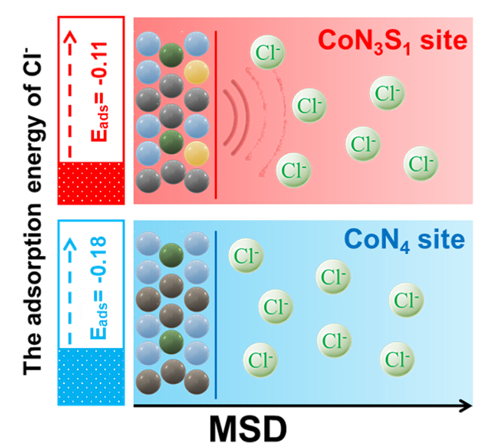
The schematic diagram of the asymmetric CoN3S1 model is more resistant to Cl- corrosion than the symmetric CoN4 model (Image by ZHANG Canhui)
The implications of this work extend beyond hydrogen production alone, as Co-N/S-HCS may be beneficial for other seawater applications, such as desalination and advanced energy storage.
“The robustness of Co-N/S-HCS opens up exciting possibilities for sustainable hydrogen production in water-scarce regions, reducing costs and minimizing environmental impact,” said Prof. HUANG Minghua of the Ocean University of China, also a corresponding author of the study.
These findings lay a strong foundation for designing seawater-resistant catalysts. The study marks a critical step forward in advancing seawater-based energy solutions and highlights the potential for sustainable hydrogen production at scale.
“We hope this work inspires further advancements in sustainable hydrogen production that can meet global energy demands,” said Prof. JIANG Heqing of QIBEBT, another corresponding author.