Researchers constructed perfectly selective artificial proton channels
Biological proton channels are a special type of ion channels, not only because they conduct a different species, but also because they have a selectivity that is far superior to other ion channels. In fact, their selectivity is considered perfect. This is required by their physiological functions---they must sieve out protons whose concentration is 6 orders of magnitude lower than common ions, such as K+. To realize such selectivity, they adopt a distinct sieving mechanism. Although they are termed “channels”, they do not really have an open channel that permit the transport of any ions or even water molecules. Instead, they are lined with hydrogen-donating groups, which, together with the surrounding water molecules in the presence of protons, form hydrogen-bonded wires on which protons can hop.
In many industrial applications including flow batteries, acid recovery, and fuel cells, such perfect proton selectivity in aqueous environment is also highly demanded. “For example, for vanadium flow battery, perfect proton selectivity of the proton exchange membrane would lead to the highest energy efficiency. For acid recovery from industrial wastewater, it is also crucial that the material can conduct protons while completely reject the permeation of toxic species” says co-author LI Qi.
“However, perfect proton selectivity in water has been challenging to realize, especially so for materials that can fabricated in bulk scale” says Prof. GAO Jun, from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), Chinese Academy of Sciences (CAS).
Now, a team led by Prof. Gao Jun and Prof. Huang Qingsong from Sichun University, found that interlayer spacings of laminar covalent organic framework (COF) materials readily serve as perfectly selectivity artificial proton channels. These materials can be easily fabricated in bulk. “Previously, the pores of the COF materials were extensively studied for proton conduction. Yet, the pore size is typically larger than 1 nm, precluding the possibility to realize perfect selectivity against ions or water molecules” says Prof. HUANG Qingsong.
Their findings were published inAngewandte Chemie International Editionon April 06 2024.
The team instead use the interlayer spacings to transport protons. The spacing is so narrow that no ions or water molecules can transport through. However, by functionalizing the COF material with hydrogen donating groups, protons can efficiently transport, exhibiting a diffusivity that is in the same order of magnitude as in bulk water. The authors used density functional theory calculations to support their findings. They showed that the proton transport mechanism is reminiscent of the biological counterparts. With the presence of hydrated protons and hydrogen-donating, hydrogen-receiving groups of the COF material, a hydrogen bonded network across neighboring COF pores is formed, allowing proton hopping. Based on such mechanism, the authors further demonstrated how they can tune the proton permeability while retaining the perfect selectivity by tuning the hydrogen-donating groups of the COF material.
"This work should provide guidance on how to design better proton-conducting materials. We are exploring methods to further scaling up the material fabrication and expect it to find applications in acid recovery or flow batteries," LI Qi adds.
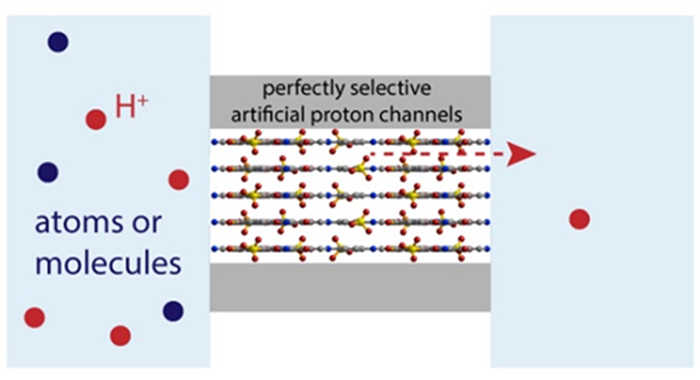
Perfectly selective proton transport through the interlayer spacings of covalent organic framework materials
(Image/Text by GAO Jun)
Contact:
KONG Fengru
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-58261072
E-mail: kongfr@qibebt.ac.cn