Scientists Propose New Strategy for Sulfide All-solid Lithium Battery Development
Sulfide solid lithium cells, which combine high energy density and excellent rate performance, are emerging as the optimal choice for powering electric vehicles. Numerous automotive companies worldwide have committed to research and development initiatives for sulfide all-solid-state lithium batteries.
According to a study published in ACS Applied Materials & Interfaces on April 8, Prof. WU Jianfei's research group from the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences addressed the industry challenges and bottlenecks in the stacking process of sulfide all-solid-state cells.
To improve the low ionic conductivity and unstable interface with the lithium anode of the Li3PS4 electrolyte, the researchers proposed a dual-element co-doping strategy to modify the Li3PS4 solid-state electrolyte. Through ball milling and low-temperature sintering, they successfully developed a novel sulfide solid-state electrolyte, Li3.04P0.96Zn0.04S3.92F0.08, which exhibited both high ionic conductivity and electrochemical stability with the lithium anode.
X-Ray Photoelectron Spectroscopy and X-ray Diffraction analyses showed that the substitution of Zn for part of P and F for part of S, forming Zn-S and Li-F bonds, respectively. The partial substitution of P5+ by the heterovalent Zn2+ facilitated an increase in lithium ion migration sites, thereby reducing the activation energy of the electrolyte and increasing its ionic conductivity.
Tests showed that the solid-state electrolytes co-doped with Zn and F exhibited an impressive ionic conductivity of 1.23×10-3 S cm-1, 3.5 times higher than the undoped counterparts, with an activation energy of 9.8 kJ mol-1 at room temperature. The introduction of F into the electrolytes resulted in a rich LiF interface with the lithium metal anode, which promoted uniform lithium ion deposition.
Conversely, the Zn-doped electrolytes formed a Li-Zn alloy with the lithium metal anode, creating a welding effect that prevented pore formation between the electrolyte and the lithium anode, thereby ensuring interface stability.
In particular, the symmetric battery comprising Li/Li3.04P0.96Zn0.04S3.92F0.08/Li anode exhibited a critical current density as high as 1 mA cm-2 and displayed stable cycling for over 500 hours at a current density of 0.1 mA cm-2.
In addition, the prepared all-solid-state Li-S cells maintained stable charge-discharge cycles for 200 cycles at 0.5 ℃ and room temperature.
This study represents a novel approach to the design of sulfide solid electrolytes and all-solid-state Li-S cells, breaks through the last barrier in the production process of sulfide all-solid-state cells for large-scale vehicle batteries, and makes a key breakthrough in the sulfide pouch type battery stacking technology.
"We are preparing for the 20 Ah sulfide all-solid-state cell molding production line by collaborating with upstream and downstream industries to expedite technology development and validation. The goal is to lead the way in realizing mass production of sulfide all-solid-state cells by 2026," said Prof. WU Jianfei.
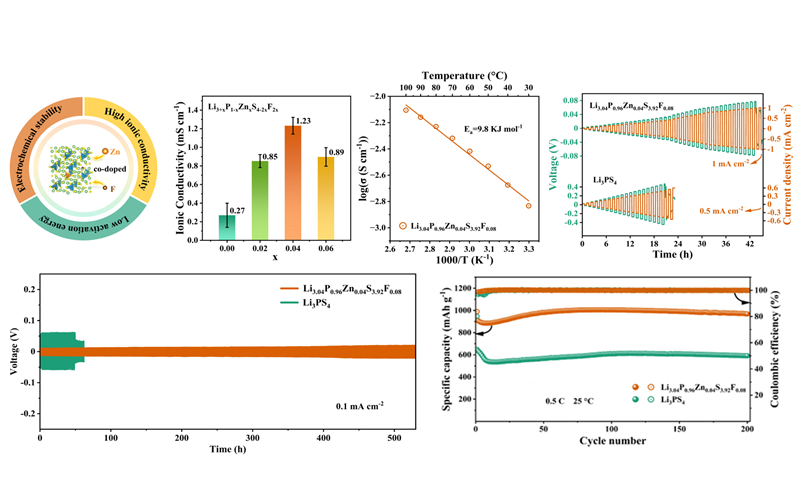
Ionic conductivity, activation energy, lithium-symmetric battery performance and all-solid-state lithium-sulfur cells performance of Zn, F co-doped electrolyte
(Image/Text by GAO Yuan, GAO Jing)
Contact:
KONG Fengru
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-58261072
E-mail: kongfr@qibebt.ac.cn