The Qingdao Institute of Bioenergy and Bioprocess Technology has recently published an important review in engineering Clostridium autoethanogenum to synthesize the biochemicals and biocommodities
The transition to fossil fuel utilization has led to serious environmental consequences, exacerbating global warming and bringing further climate change. In the face of these challenges, multiple approaches are needed to develop alternative sustainable chemicals and fuels. In this context, microbial biocatalysis synthesis processes have attracted wide attention. Self-produced ethanol Clostridia is a model organism for microbial gas fermentation. In particular, recent development of synthetic biology tools for this strain has enabled us to better understand its unique physiological characteristics and achieve engineering transformation.
Qingdao C1 refinery engineering research center, QingDao institute of bioenergy and bioprocess technology has published a review article, which summarized and prospected the metabolic engineering of self-produced ethanol Clostridia for the synthesis of chemicals and biocommodities from syngas.
This work was published in Synthetic and Systems Biotechnology on 15 December 2023.
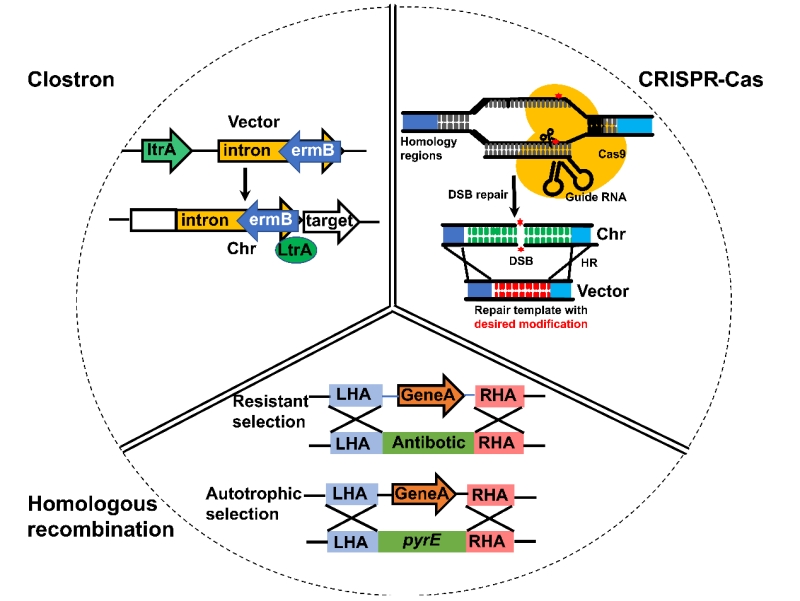
This review summarizes progress in C. autoethanogenum genome editing tools research, the special energy metabolism model of autotrophic metabolism, and progress in metabolic engineering. C. autoethanogenum is a promising platform for sustainable production of bulk chemicals, but its engineering transformation still needs to address the following issues: (1) To further understand the basic biology of this strain, high-throughput functional genomics tools need to be developed to study gene interaction networks, giving new insights into the genotype-phenotype relationship. (2) In terms of engineering biology, the metabolic engineering of self-produced ethanol Clostridia is limited by its energy demands, and computer-aided methods have significant advantages in metabolic pathway design and enzyme engineering. (3) Recent developments in synthetic biology component tools, such as DNA genetic component libraries and the construction of genetic regulatory circuits, can further optimize the expression of synthetic pathways and balance metabolic flux.
(Image/Text by TAN Yang)
Contact:
KONG Fengru
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-58261072
E-mail: kongfr@qibebt.ac.cn