"Dual-ligand" Strategy Helps to Achieve Perfect Asymmetric Kinetic Resolution Polymerization
Benefiting from unique and excellent properties, study on synthesis and application of chiral materials has important scientific significance and market value. However, the design, synthesis and application of chiral materials are still in their infancy, and relevant research is limited to natural chiral polymer materials and very few artificial synthetic chiral materials.
In response to the key problems of synthesis, characterization, and mechanism research in the field of chiral materials, researchers from the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences have proposed a novel concept of "asymmetric kinetic resolution polymerization" (AKRP), which provides a new method for the efficient synthesis, direct characterization of chiral polymer materials, and a new approach for studying the reaction mechanism of asymmetric polymerization.
The challenge to achieve AKRP is the design and synthesis of highly enantioselective catalysts. To solve this problem, the researchers proposed the "dual-ligand" strategy for the first time, enabling the highly enantioselective chiral (BisSalen)Al catalysts.
Results were published in Journal of the American Chemical Society on March 1.
In this study, the researchers constructed a novel chiral (BisSalen)Al catalyst by introducing a second ligand into a typical (Salen)Al catalyst. The chiral (BisSalen)Al catalyst processes extremely high enantio-discrimination performance, resulting in polymer preference for the monomer with a certain configuration, but no recognition for the monomer with another configuration.
The experimental results show that the chiral (BisSalen)Al complex exhibits excellent enantioselectivity during the AKRP process, with an enantiomeric excess (ee) of the unreacted monomer exceeding 99% and a kinetic resolution coefficient krel exceeding 500, making it the first perfect AKRP catalyst among racemic six-membered glycolides.
The detailed nuclear magnetic resonance analysis and density functional theory theoretical calculation proved that the "dual-ligand" design constructed a more confined asymmetric microenvironment formed by dual ligands, which further improved the enantio-discrimination performance of (BisSalen)Al catalyst, thus achieving a highly enantioselective AKRP process.
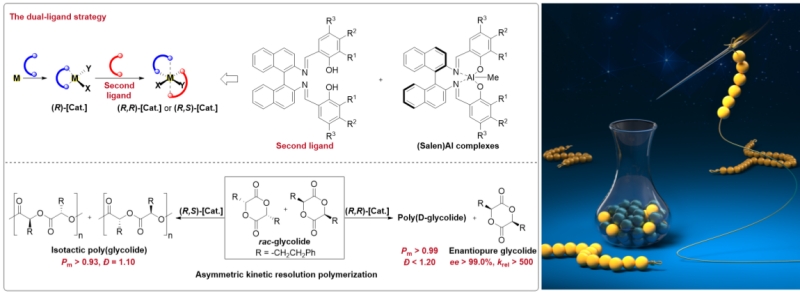
Left: The “dual-ligand” strategy designed Bis(Salen)Al catalysts for the AKRP of racemic phenethylglycolide. Right: The schematic diagram of asymmetric kinetic resolution polymerization
(Image/Text by GUO Xuanhua and XU Guangqiang)
Contact:
KONG Fengru
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-58261072
E-mail: kongfr@qibebt.ac.cn