B-site Rock-salt-ordered Cu-based Double Perovskite Realizes High Efficiency and Stable CO2 Electroreduction
Carbon dioxide electroreduction (CO2RR) into high-value chemical feedstocks and fuels is a potential way to realize the carbon-neutral cycle. Cu-oxide-based catalysts are promising for CO2 electroreduction, but suffer from inevitable reduction and structural collapse, leading to unstable electrocatalytic properties.
Profs. ZHU Jiawei and JIANG Heqing from the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences have developed a B-site rock-salt-ordered double perovskite oxide of Sr2CuWO6 with superexchange-stabilized long-range Cu sites for efficient and stable CO2 electroreduction.
The study was published in Nature communications on Feb. 21.
The researcher used Sr2CuWO6 as a proof-of-concept model catalyst. The Sr2CuWO6 exhibited B-site rock-salt order, resulting in long Cu-Cu distance and superexchange interaction.
Its long-distance Cu sites facilitate *CO hydrogenation and inhibit C-C coupling. Meanwhile, the superexchange interaction stabilizes the Cu sites and prevents structural collapse. These factors realized the excellent performance of Sr2CuWO6 for stable CO2 electromethanation, which achieved a high FECH4 of 73.1% as well as a high partial current density of 292.4 mA cm-2.
Notably, Sr2CuWO6 presents the best performance of electromethanation in perovskite catalysts.
This work discovers efficient and stable Cu-based double perovskite oxide for CO2RR, which opens a new avenue for rational design of more advanced Cu-based catalysts.
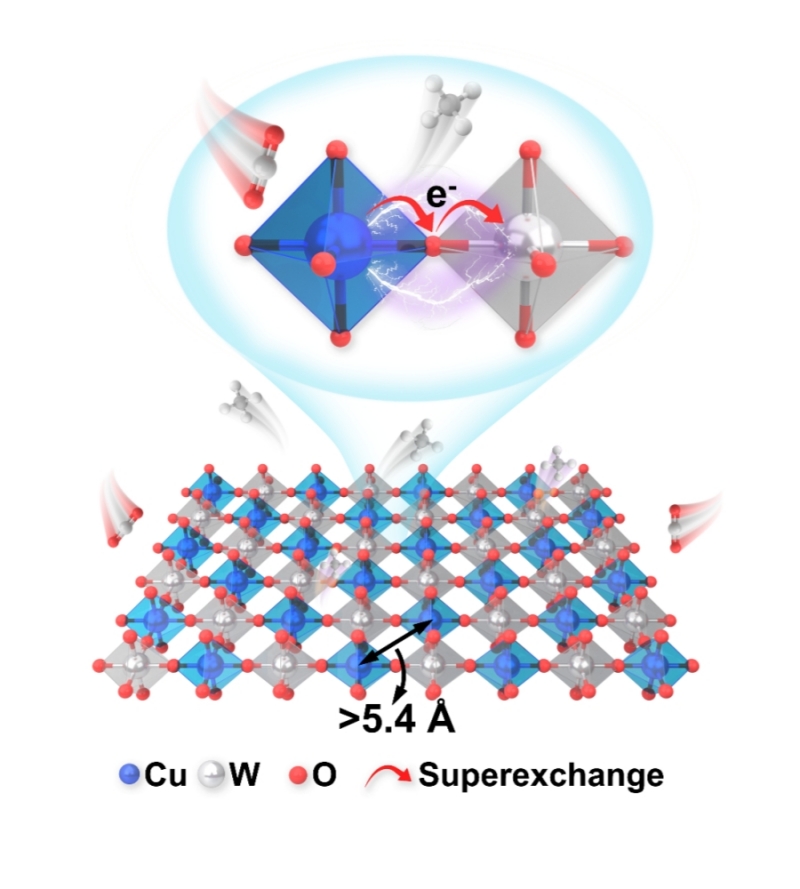
The schematic diagram of B-site rock-salt order Sr2CuWO6 with long Cu-Cu distance and superexchange interaction for electromethanation
(Image/Text by Yu Zhang)
Contact:
KONG Fengru
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-58261072
E-mail: kongfr@qibebt.ac.cn