Researchers Succeed in Converting Carbon Dioxide into Glucose Utilizing Cyanobacteria
Glucose is the most abundant monosaccharide, serving as an essential energy source for cells and as an important feedstock for the biorefinery industry. The plant-biomass-sugar route dominates the current glucose supply, while it is influenced by multiple parameters, such as plant-cultivation cycles, biomass-collection radius, and pre-treatment costs.
Against the backdrop of global climate crisis and worsening food shortages, developing more efficient, continual, and industrial glucose production routes is needed. However, direct conversion of carbon dioxide into glucose through natural photosynthesis process has not made a breakthrough for a long time.
Recently, a research team led by Prof. LU Xuefeng and Prof. LUAN Guodong from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), Chinese Academy of Sciences (CAS), identified key factors restricting natural potential of cyanobacteria to directly convert carbon dioxide into glucose and constructed efficient glucose synthesizing photosynthetic cell factories.
The study was published in Nature Communications on June 9.
In photoautotrophs, e.g., higher plants and algae, glucose is synthesized as storage for carbon and energy. Glucose metabolism possesses complex interactions with photosystems, disturbs the synthesis and metabolism of pigments, and may even inhibit photosynthetic activities. Therefore, free glucose is rarely synthesized or accumulated in excess in photosynthetic cellular metabolism.
The research team utilized an important cyanobacterium Synechococcus elongatus PCC 7942 as the chassis and identified native glucokinase activity as the bottleneck restricting metabolism potential for glucose synthesis.
Targeted knockout of two glucokinase genes disturbed the carbohydrate metabolism and activated a metabolic flux towards glucose through the sucrose metabolism network. The enhanced glucose synthesis promoted the enrichment of a specific spontaneous genomic mutation on the chromosome of PCC 7942, which facilitates efficient glucose secretion.
"These findings underline the cyanobacterial metabolism plasticities and demonstrate their potential for direct photosynthetic production of glucose," said Prof. LUAN.
The researchers further clarified the pathways and mutations leading to glucose synthesis and secretion and optimized the recombinant strains' glucose synthesis performances. Through subsequent metabolic engineering and cultivation optimization, the glucose secreted by the engineered strain surpasses 5 g/L during long-term cultivation, accounting for up to 70% of the fixed carbon source.
"This is the first successful attempt to construct a direct link between natural photosynthesis and stable glucose synthesis," said Prof. LU. "Our findings shed light on developing and industrializing more directional and continuous glucose production systems with solar energy and carbon dioxide."
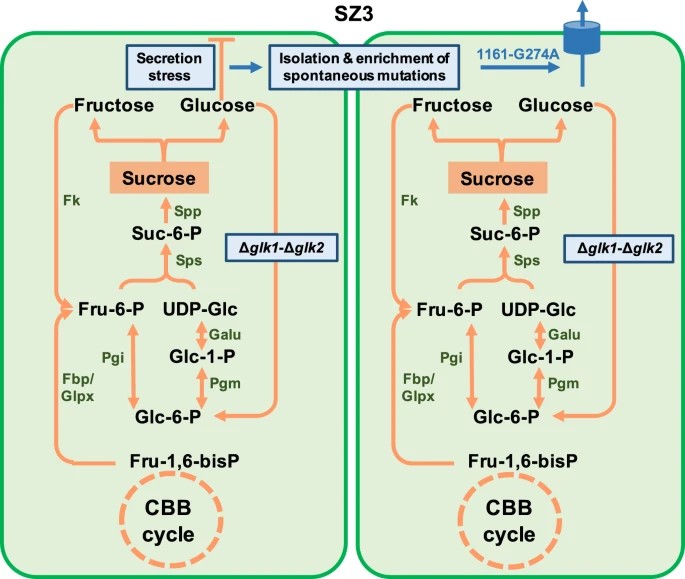
Schematic diagram of genome mutation and glucose secretion mechanism promoted by glucokinase deficiency. (Imaged by LUAN Guodong)
(Text by LUAN Guodong)
Contact:
KONG Fengru
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-58261072
E-mail: kongfr@qibebt.ac.cn