Scientist Reveal Void-Confinement Effects of Hollow Nanoreactors
Hollow nanoreactors have attracted great interest in catalysis research due to their unique catalytic properties, especially the void-confinement effects. During the past decades, many studies have been devoted to unraveling the void-confinement effects of hollow nanoreactors in liquid-phase reactions.
However, in the catalytic process, there are many factors that affect the catalytic performance. Especially in the liquid phase hydrogenation reaction, the catalyst structure and reaction conditions will have a significant impact on the catalytic performance.
Therefore, providing both of comparative nanomaterials and reaction system with consistent reaction conditions to decouple the void-confinement effects from other factors is highly required to unambiguously ascribe and understand the void-confinement effects of the hollow nanoreactors.
Recently, Prof. WANG Guanghui from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LIU Jian from the Dalian Institute of Chemical Physics (DICP) of CAS, reported a general strategy to synthesize a pair of hollow carbon sphere (HCS) nanoreactors with pre-synthesized PdCu nanoparticles encapsulated inside of HCS (PdCu@HCS) and supported outside of HCS (PdCu/HCS), respectively, while keeping other structural features the same. Based on the two comparative nanoreactors, void-confinement effects in liquid-phase hydrogenation are investigated in a two-chamber reactor.
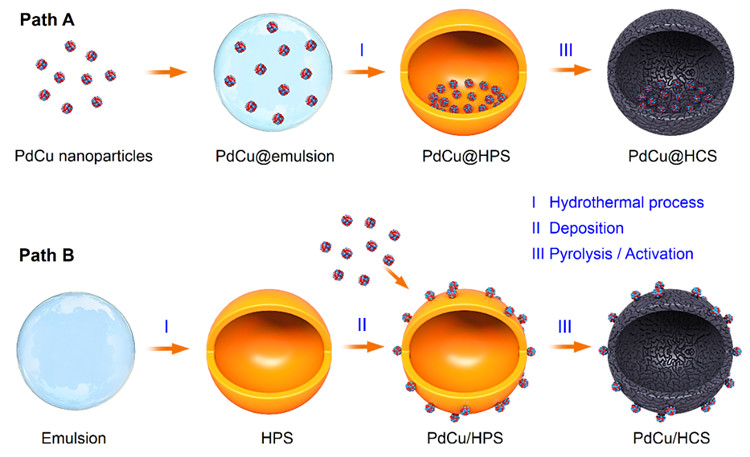
Schematic illustration for the synthesis of PdCu@HCS and PdCu/HCS. (Image by DONG Chao)
"It has found that, compared with PdCu/HCS, the PdCu@HCS can accelerate the hydrogenation of styrene via an accumulation of reactant molecules, decelerate the hydrogenation of 2-vinylnaphthalene due to the mass transfer limitation and inhibit the hydrogenation of 9-vinylanthracene because of the molecular-sieving effect," said Prof. WANG.
In addition, the void space of the PdCu@HCS can alter the hydrodynamics of the intermediates, and correspondingly change the catalytic selectivity during hydrogenation of small alkynes. Moreover, a specific imine has been selectively produced over the PdCu@HCS by utilizing the shape-selective catalysis principle.
"These studies provide straightforward examples for clearly understanding and estimating the void-confinement effects of the hollow nanoreactors in liquid-phase hydrogenations," said Prof. LIU.
Moreover, various pairs of hollow nanoreactors can be designed based on the synthesis strategy, and employed as ideal models for study of catalytic mechanisms in various reactions, and then guiding the design of efficient catalysts for specific chemical transformations and the development of nanoreactor reaction engineering.
This study was published in Angewandte Chemie International Edition. It was supported by National Natural Science Foundation of China, QIBEBT and the DNL Cooperation Fund, CAS.
(Text by DONG Chao and YU Qun)
Contact:
CHENG Jing
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
E-mail: chengjing@qibebt.ac.cn