New Strategy for CO2 Electrochemical Reduction
Carbon dioxide (CO2) emission has become a global problem, leading to the greenhouse effect greenhouse to the earth. Hence, efficient conversion of CO2 into value-added liquid fuels is one of significant ways to fix CO2, and it can alleviate the growing shortage of non-renewable fossil fuels at the same time.
The electrochemical reduction of CO2 to value-added products obtains great attention and investigation worldwide in recent years owing to its mild reaction conditions and high energy efficiency. However, it remains a challenge to achieve a large current density with a high Faraday efficiency.
Recently, a research team led by Prof. LIU Licheng from Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), Chinese Academy of Sciences (CAS), found that directly coating the amino-functionalized carbon layer can effectively regulate the electronic structure of the non-precious metal catalyst SnS, thereby accelerating the electron conduction efficiency and enhancing the adsorption energies of OCHO* and CO2*, which leads to an increase in reaction current density and formate production.
This work provides a new strategy for improving the catalytic activity of transition metal catalysts for CO2 electrocatalytic reduction.
The related progresses were published in Advanced Energy Materials. This work was supported by the National Natural Science Foundation of China and Dalian National Laboratory For Clean Energy Cooperation Fund.
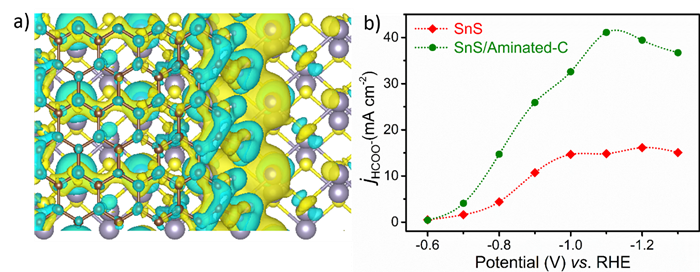
Fig.1: The differential charge diagram of SnS/Aminated-C for (a) and the partial current density of formate production for SnS and SnS/Aminated-C for (b). (Image by CHEN Zhipeng)
(Text by CHEN Zhipeng)
Contact:
CHENG Jing
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-80662647/80662622
E-mail: chengjing@qibebt.ac.cn