Continuous increasing pressures of fuel production and environmental protection have led to surge in the search for alternative and renewable energy. Many species of microalgae produce amounts of lipids, especially triacylglycerides (TAGs), the best feedstock for biodiesel production.
Yellow-green microalga Tribonema sp. is the first reported filamentous oleaginous microalgae. It has advantages over other unicellular oily microalgae for cultivation adaptability and easy harvesting due to its filamentous character. Moreover, Tribonema sp. is also capable of using glucose as substrate for rapid heterotrophic growth, providing a way to speed up the production of microalgal lipid.
Previous study showed that although filamentous microalgae T. minus can accumulate lipids up to 62% of the dry cell weight under photoautotrophic condition, however, the lipid content decreased dramatically under heterotrophic condition. Understand the mechanism differences in metabolic pathways between photoautotrophic and heterotrophic growth will provide strategies for promoting lipid accumulation in heterotrophy.
Recently, Microalgae Biotechnology Group led by Prof. LIU Tianzhong from Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), Chinese Academy of Sciences (CAS), studied the metabolic differences of lipid accumulation between photoautotrophically and heterotrophically cultivated T. minus cells by comparing the growth, biochemical composition and transcriptomic and metabolomic profiles of the cells, and they constructed a global network model of the central carbon metabolism and lipid biosynthetic pathways of about two cultivation modes. The results showed that suppressed lipid accumulation heterotrophically cultivated T. minus might result mainly from down-regulation of glycolysis, de novo fatty acids and lipid biosynthesis, and up-regulation of gluconeogenesis. Thus, the insufficient supply of carbon precursors related fatty acid synthesis caused low levels of final lipid accumulation during heterotrophic cultivation. They proposed an effective strategy to supplement exogenous carbon metabolites, especially potassium palmitate, to dramatically enhance the total lipid content in T. minus grown in heterotrophic culture.
The related work was published in Biotechnology for Biofuels.
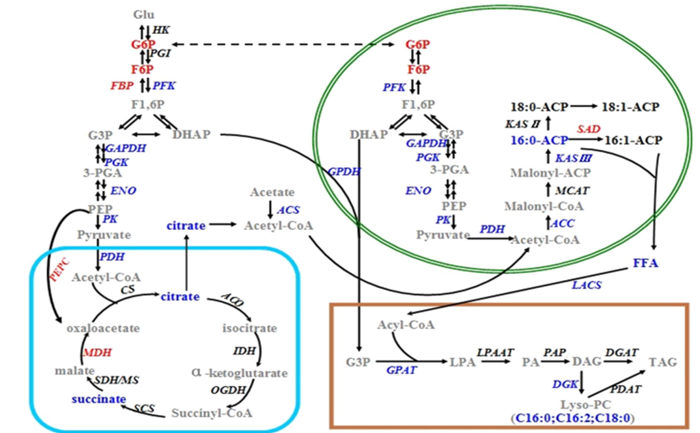
Figure 1.Reulatory model for the flow pathway of central carbon metabolism and a proposed scheme for lipid biosynthesis in T. minus. (Image by WANG Hui)
(Text by WANG Hui and LIU Tianzhong)
Contact:
CHENG Jing
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-80662647
E-mail: chengjing@qibebt.ac.cn