Cellulose is the earth’s most abundant biopolymer which is responsible for the structural integrity of plants. Processive cellulases are essential in crystalline cellulose degradation as they continuously catalyze and slide along the cellulose chain before dissociating.
There are two types of processive cellulases: exoglucanases and processive endoglucanases. The working mode of exoglucanases was named as threading mode. Whereas, the working mode of processive endoglucanases hasn’t been proposed since their open cleft in the catalytic module.
Recently, a research team led by Prof. LI Fuli from Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), Chinese Academy of Sciences (CAS), proposed a wirewalking mode for processive degradation of crystalline cellulose by an endoglucanase (Fig. 1), which provided insights for rational design of industrial cellulases.
CcCel9A is a processive endoglucanase from the bacterium Clostridium cellulosi, comprises a glycoside hydrolase (GH) family 9 module with an open cleft and five carbohydrate-binding modules (CBMs). The results demonstrate that the C-terminal CBM3b and three CBMX2 modules enhance the productive adsorption to substrate, while the CBM3c adjacent to the GH9 is tightly bound to 11 glucosyl units, thereby extending the catalytic cleft to 17 subsites, which facilitates decrystallization by forming a supramodular binding surface. In the open cleft, the strong interaction force between substrate-binding subsites and glucosyl rings enables cleavage of the hydrogen bonds and extraction of the single cellulose chain. In addition, subsite ?4 is capable of drawing the chain to its favored location. Cellotetraose is released from the open cleft as the initial product to achieve high processivity, which is further hydrolyzed to cellotriose, cellobiose and glucose by the catalytic cleft of the endoglucanase.
"CcCel9A recruits multiple modules, whereby the GH9 and CBM3c together form an open cleft and four product-releasing glucosyl sites, consisting of up to 17 glucosyl binding subsites, to achieve processivity," said Prof. LI.
The related work was published on Biomacromolecules. The research was supported by the National Natural Science Foundation of China and the Shandong Province Natural Science Funds for Distinguished Young Scholars.
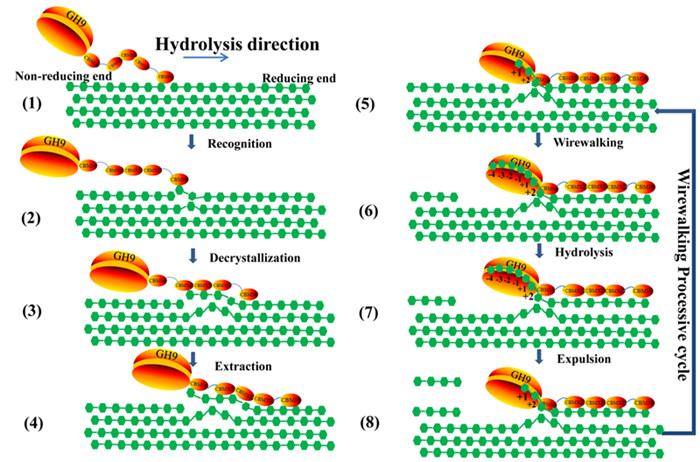
Figure 1. Working model of CcCel9A. (Image by ZHANG Kundi)
(Text by ZHANG Kundi)
Contact:
Prof. LI Fuli, Ph.D,
Qingdao Institute of BioEnergy and Bioprocess Technology (QIBEBT), CAS
Tel: 86-532-80662655
E-mail: lifl@qibebt.ac.cn