Proteins are essential parts of organisms and participate in virtually every process within cells. There are many non-covalent interactions in protein which are critical to protein structure and function. Van der Waals force is a kind of non-covalent interaction which cannot be ignored.
Van der Waals forces include attraction and repulsions between atoms, molecules, and surfaces, they are caused by correlations in the fluctuating polarizations of nearby particles. They are weaker but numerous, so they are non-negligible in proteins.
The J-coupling transmitted across the van der Waals interaction has been studied computationally, although, to the best of our knowledge, no such measurement has been reported in biomolecules.
Protein GB3, which has 56 amino acids, was used as a test protein for the measurement. We used a 1H-13C HMQC sequence modified from an HSQC method which has a more favorable relaxation property for the methyl group. To slow down the spin relaxation, the methyl groups of isoleucine (I), leucine (L), and valine (V) were [13C,1H] labeled and their neighboring aliphatics were [12C,2H] labeled whereas all other residues were [13C,2H] labeled. This method allowed us to build up the antiphase Carbon (methyl)? Carbon (aliphatic) (CmetCali) magnetization through a long 2T delay . A total of 11 vdwJCmetCali with values ~0.1?0.6 Hz were extracted for unambiguously assigned nonoverlapping CmetCali peaks. Quantum mechanical calculations suggest that the J-coupling is correlated with the exchange-repulsion term of van der Waals interaction.
This work is the first study detecting the J-coupling across the CH/CH van der Waals interaction in proteins. Nevertheless, the direct detection of JCC coupling provides an opportunity to study van der Waals interaction in greater detail in small and medium sized proteins. NMR detection of vdwJCC-coupling offers a new tool to characterize such interactions in proteins.
This work was supported by National Key R&D Program of China (Grant No. 2017YFA0505400 to L.Y.) and the National Natural Science Foundation of China (Grant Nos. 31661143036 to L.Y., 31600051 to Y.W.). A portion of this work was performed on the Steady High Magnetic Field Facilities, High Magnetic Field Laboratory, Chinese Academy of Sciences.
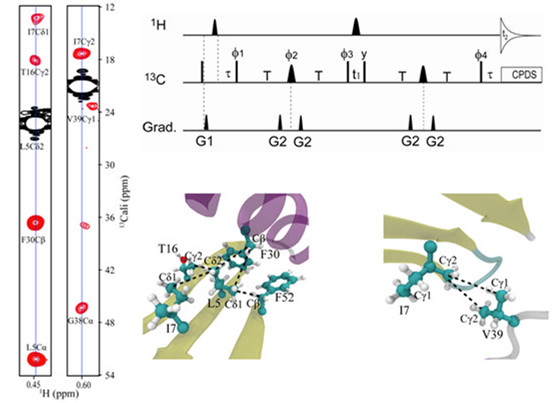
Figure 1. Detection of through-space vdwJCmetCali coupling between carbons of methyl and aliphatic groups. (Image by LI Jingwen)
(Text by LI Jingwen)
Contact:
Prof. YAO Lishan Ph.D
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
E-mail: yaols@qibebt.ac.cn