Temperature is one of the most important factors for evolution. As the temperature increases, the catalytic activity of the enzyme increases correspondingly, but at the same time, the affinity between the enzyme and the substrate decreases. It has been proved that metal ions enhance the activity, stability and substrate affinity of metal enzymes. However, the role of metal ions-binding amino acids in thermophilic adaptation is not clear.
Recently, a research team led by Prof. LI Fuli from Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), Chinese Academy of Sciences (CAS), unraveled the function of metal ions-binding amino acids in improving substrate affinity.
The researchers chose a novel thermophilic bacterium, Defluviitalea phaphyphila, which is isolated from coastal sediment. It exhibited an intriguing ability to degrade polysaccharides of brown algae, including alginate, and laminarin. A Ca2+ dependent alginate lyase AlgAT0 was characterized and in the presence of Ca2+, the optimum temperature of AlgAT0 was 75°C, and the half-life was as long as 4.5 hours, which was much higher than the reported thermostable alginate lyases previously.
Furthermore, the researchers studied the roles of calcium-binding amino acids in thermophilic adaption. Sequence alignment revealed that in one of the four calcium-binding sites AlgAT0 used aspartic acid (D) instead of glutamic acid (E) (Figure 1). Since β-COOH of aspartate showed a lower dissociation constant than γ-COOH of glutamate, the researchers hypothesized that aspartate might bind Ca2+ tighter than glutamate. Isothermal titration calorimeter showed that the calcium affinity of AlgAT0 was indeed higher than that of the mutant D238E. Further enzyme kinetics studies confirmed that both the substrate affinity and the optimum temperature of mutant D238E decreased, but the protein stability did not change indicating that the substitution of aspartate for glutamate increased the calcium binding affinity, which then contributed to the tight binding of substrate at elevated temperatures.
This study not only provides new insights into the mechanism of high temperature adaption, but also sheds new light on the directed evolution of metal ion-containing enzymes. The related findings were published in FEBS letters (DOI: 10.1002/1873-3468.12965). The research was supported by the National Natural Science Foundation of China and the Shandong Province Natural Science Funds for Distinguished Young Scholars.
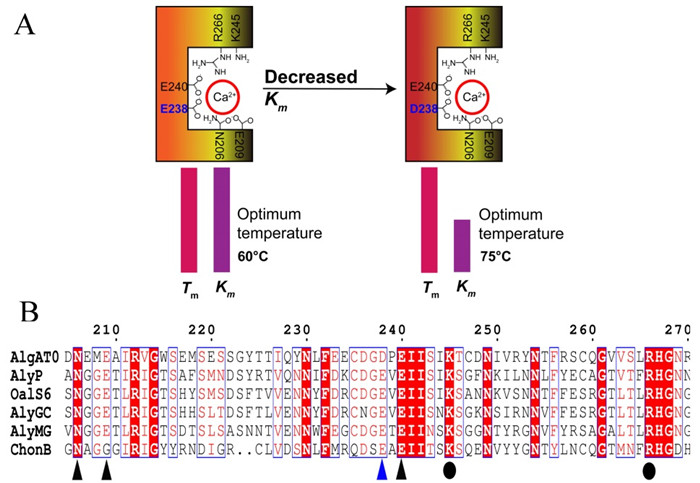
Figure 1 A. Substitution of aspartate to glutamate increased substrate binding affinity but did not change protein stability. Km, Michaelis-Menten constants. Tm, melting temperatures. B. Protein sequence alignment. Triangles and circles indicate the proposed calcium-binding sites and catalytic sites, respectively. (Image by WANG Bing)
(Text by MA Xiaoqing and WANG Bing)
Contact:
Prof. LI Fu-Li, Ph.D.,
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-80662655
E-mail: lifl@qibebt.ac.cn