Two Modification Hydrolases “Speed-Up” Biomass Utilization
Biomass is the most abundant bio-resource on earth. Its utilization enrolls in the central step of “Carbon Cycle” in nature, and now offers the renewable materials for modern industry. After billions years evolution, plant cell walls formed a natural degradation–resistant barriers. A research group in Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), Chinese Academy of Sciences (CAS), reported two modification glucoside hydrolases, which were hyperthermophilic-tolerant, to accelerate biomass degradation.
In a thermophilic bacterial species Caldicellulosiruptor, two α-L-arabinofuranosidases (XynF and AbF51) were identified. XynF was a “master” of arabinose-degradation, which hydrolyzed the α-1,2-, α-1,3-, and α-1,5- L-arabinofuranosyl bonds in arabinose-based polysaccharides. Combined with endo-xylanase XynA, XynF improved arabinoxylan hydrolysis by more than 6-fold compared to the level seen with XynA alone. Another one, AbF51, cleaved the α-1,5-L-arabinofuranosyl glycoside bonds. Thus, XynF and AbF51 processed the bioconversion of arabinose-containing oligosaccharides to fermentable saccharides (Figure 1).
Moreover, a thermostable lichenase F32EG5 from Caldicellulosiruptor sp. F32 was also characterized. F32EG5 cleaved the β-1,4 linkage or the β-1,3 linkage while a 4-O-substitued glucose residue linked to a glucose residue through a β-1,3 linkage (Figure 2A), which was completely different from extensively studied GH16 lichenase that catalyzes strict endo-hydrolysis of the β-1,4-glycosidic linkage adjacent to a 3-O-substitued glucose residue in the mixed linked β-glucans. Structure of the complex of F32EG5 E193Q mutant and cellotetraose revealed a sharply-bent substrate conformation that fitting to the bent sugar chains in lichenan (Figure 2B). The mechanism of thermostability and substrate selectivity of F32EG5 was clearly demonstrated by molecular dynamics simulation and site-directed mutagenesis.
The related findings were published in Applied and Environmental Microbiology and Biochemical Journal. The work were supported by the National Natural Science Foundation of China, the Shandong Province Natural Science Funds for Distinguished Young Scholar and the Key Scientific and Technological Project of Shandong Province. These work were collaborated with Prof. Robert Kelly from the North Carolina State University and researchers from Tsinghua University.
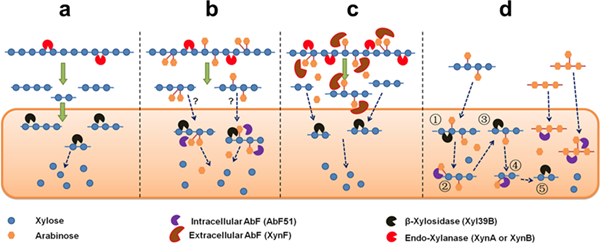
Figure 1 Bio-degradation mechanism of two distinct arabinofuranosidases from Caldicellulosiruptor (Image by MENG Dongdong)
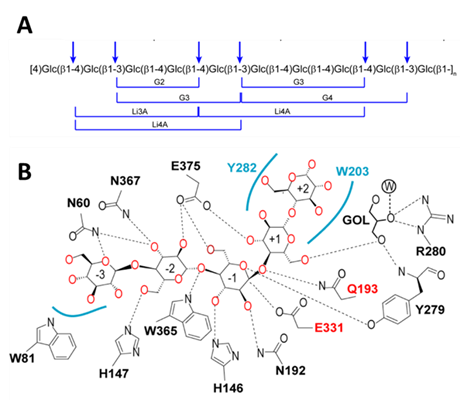
Figure 2 Substrate specificity of GH5 lichenase F32EG5. (A) The proposed cleavage sites and possible products of barley β-glucan hydrolysed by F32EG5. (B) Schematic diagram of the G5 recognized by F32EG5-E193Q. (Image by MENG Dongdong)
(Text by LU Ming and MENG Dongdong)
Contact:
Prof. LI Fu-Li, Ph.D.,
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences
Tel: 86-532-80662655
E-mail: lifl@qibebt.ac.cn