QIBEBT Reported Novel Substrate-specific Recognition Mechanisms
As the most abundant renewable resource on earth, lignocellulosic biomass is involved in the central step of “Carbon Cycle” in nature. After billions years evolution, plant cell walls form a natural degradation–resistant barriers.
The Microbial Resource Group in Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), CAS, focuses on the mechanistic studies of thermophilic microorganism-participant lignocellulosic biodegrading system.
In a recent paper published in the journal of Applied Environmental Microbiology, all the putative α-L-arabinofuranosidases (AbFs) in Caldicellulosiruptor species were screened. From this screen, an extracellular XynF was determined to be a key factor in hydrolyzing α-1,2-, α-1,3-, and α-1,5-l-arabinofuranosyl residues of arabinose-based polysaccharides. Combined with a GH11 xylanase (XynA), XynF increased arabinoxylan hydrolysis more than 6-fold compared to the level seen with XynA alone.
These results demonstrated the separate but complementary contributions of extracellular XynF and cytosolic AbF51 in processing the bioconversion of arabinose-containing oligosaccharides to fermentable monosaccharides (Figure 1). This work was collaborated with Prof. Robert Kelly from the North Carolina State University. A China Patent was also submitted.
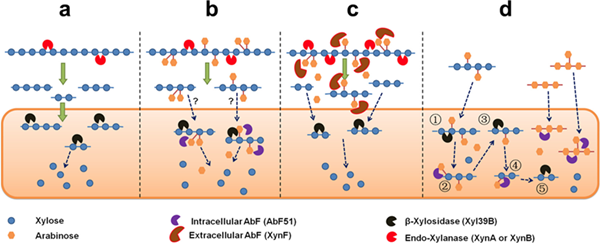
Figure 1 Bio-degradation mechanism of two distinct arabinofuranosidases from Caldicellulosiruptor (Image by MENG Dongdong)
In another work published in Biochemical Journal the crystal structure of a thermostable lichenase F32EG5 from Caldicellulosiruptor sp. F32 was resolved by a joint research team from QIBEBT and Tsinghua University. F32EG5 cleaved the β-1,4 linkage or the β-1,3 linkage while a 4-O-substitued glucose residue linked to a glucose residue through a β-1,3 linkage (Figure 2A), which was completely different from extensively studied GH16 lichenase that catalyzes strict endo-hydrolysis of the β-1,4-glycosidic linkage adjacent to a 3-O-substitued glucose residue in the mixed linked β-glucans. Structure of the complex of F32EG5 E193Q mutant and cellotetraose revealed a sharply-bent substrate conformation that fitting to the bent sugar chains in lichenan (Figure 2B).

Figure 2 Substrate specificity of GH5 lichenase F32EG5. (A) The proposed cleavage sites and possible products of barley β-glucan hydrolysed by F32EG5. (B) Schematic diagram of the G5 recognised by F32EG5-E193Q. (Image by MENG Dongdong)
This study was supported by the National Natural Science Foundation of China, the Shandong Province Natural Science Funds for Distinguished Young Scholar and the Key Scientific and Technological Project of Shandong Province.
(Text by LU Ming and MENG Dongdong)
Contact:
Prof. LI Fuli, Ph.D,
Qingdao Institute of BioEnergy and Bioprocess Technology (QIBEBT), CAS
Qingdao, Shandong 266101, China,
Tel: 86-532-80662655
E-mail: lifl@qibebt.ac.cn
http://mr.qibebt.ac.cn