QIBEBT Developed Biomimetic Oligopeptide-directed Self-assembly of Functional Molecules
Biomacromolecules, such as deoxyribonucleic acid (DNA), protein, and carbohydrate etc., can form well-organized, high ordered nanostructures, which plays an important role in the functionalization of organisms. The construction of biobased materials by taking advantage of the well-organized hierarchical nanostructures and the biocompatibility of biomolecules has been attracting much attention.
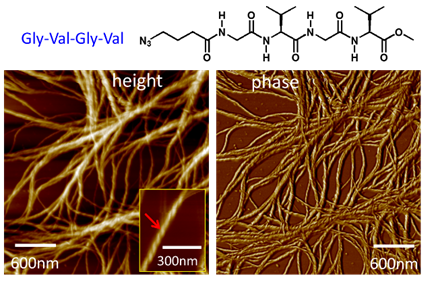
Prof. WAN Xiaobo’s group from CAS Key Laboratory of Biobased Materials has synthesized biomimetic oligopeptides which can form b-sheet structures and further investigated the peptide-directed self-assembly of functional molecules, especially the conjugated semiconductors. The results have been published in Supramolecular Chemistry, Macromolecular Chemistry and Physics, and Soft Matter. Figure 1. Gly-Val-Gly-Val and the chiral fibers
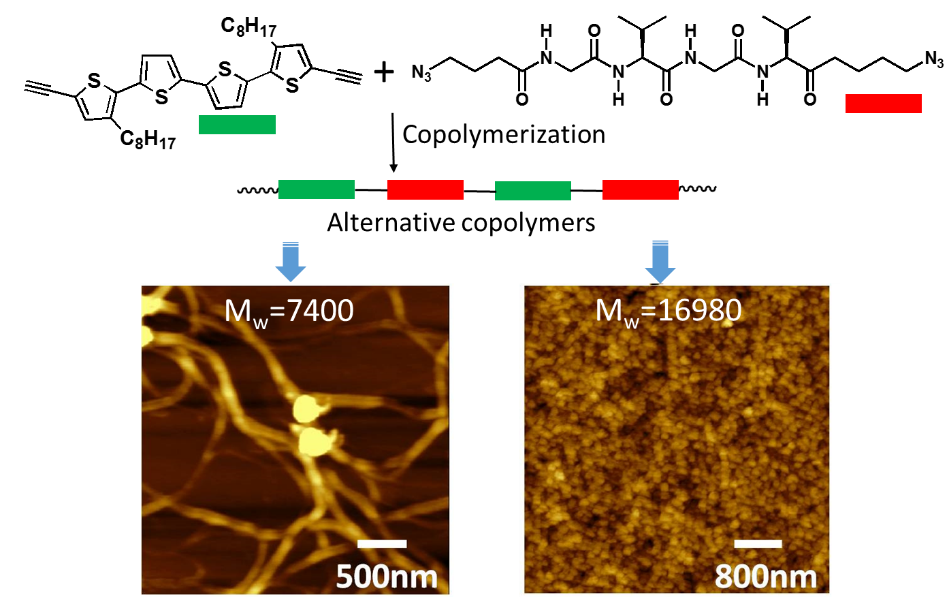
Gly-Val-Gly-Val and quaterthiophene, the nanostructures can be manipulated by changing the molecular weight, low-molecular-weight polymer assembled into thick fibers, and the high-molecular-weight polymers formed spherical particles (Figure 4, Macromolecular Chemistry and Physics, 2014, 215(9), 906-914).
 |
 |
|
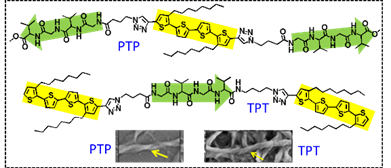
Figure 2. Conjugates of Gly-Val-Gly-Val with oligothiophene | |
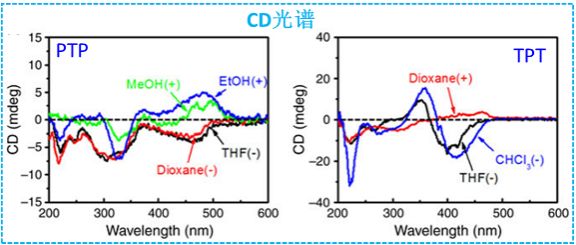
The conjugate of Gly-Val-Gly-Val peptide and dithienylcyclopentene was also prepared. It forms an organogel in Tetrahydrofuran (THF). This organogel is multi-responsive to various external stimuli including temperature, light, chemical, and mechanical force. Moreover, in the presence of catechol, this gelator forms a more robust organogel, accompanied with a dramatic change of the assembly manner and rheological properties (Figure 3, Soft Matter, 2013, 9, 7538-7544).
|
|
|
Figure 3. Conjugates of Gly-Val-Gly-Val with dithienylcyclopentene |
 |
|
Figure 4. Alternative copolymers of Gly-Val-Gly-Val and quaterthiophene |
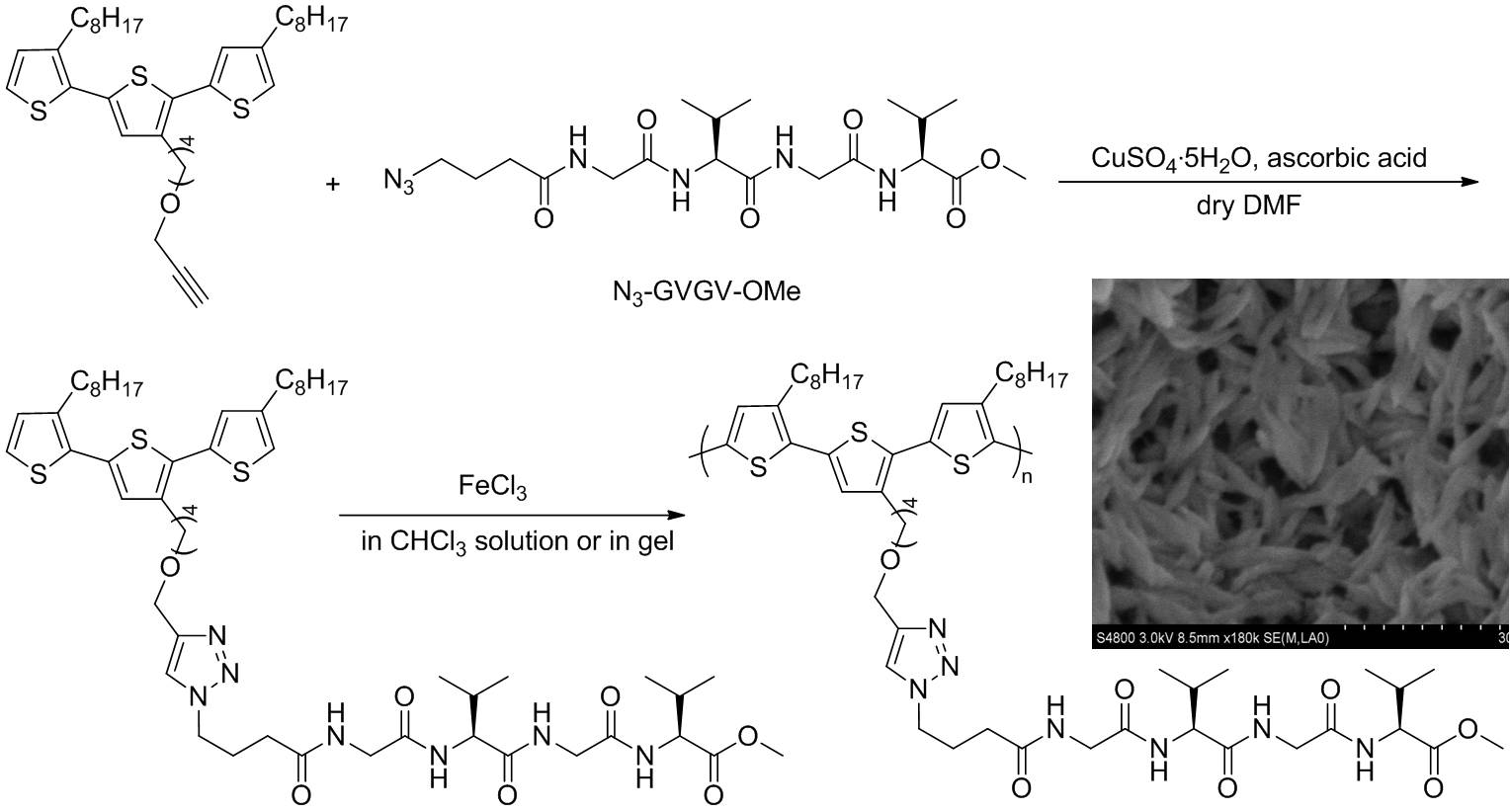 |
|
Figure 5. Polythiophene decorated with Gly-Val-Gly-Val as side chains |
Reference:
1. Supramolecular Chemistry2013, 25(5), 269-275.
2. Supramolecular Chemistry2014, 26(5-6), 383-391.
3. Soft Matter2013, 9, 7538-7544.
4. Macromolecular Chemistry and Physics 2014, 215(9), 906-914.
5. Supramolecular Chemistry2013, 25(12), 842-847.
Contact:
Prof. WAN Xiaobo
Email: wanxb (AT) qibebt.ac.cn
Web: http://english.qibebt.cas.cn