QIBEBT reveals the contribution of cellulosomal scaffoldins to cellulose hydrolysis by Clostridium thermocellum
Clostridium thermocellum is known as the most efficient cellulose-degrader in nature. However, due largely to lack of efficient methods for genetic manipulation of C. thermocellum, in vivo function of its cellulosome system is rarely studied. Metabolomics Group of Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) is working towards the study on C. thermocellum and its cellulosome for long term, and has analyzed the intracellular functions of all scaffoldin proteins in C. thermocellum using a newly developed thermotargetron system (Mohr G, Hong W, et al, PloS One 8(7):e69032). The results have been published in Biotechnology for Biofuels (Hong W, et al, Biotech Biofuels.2014, 7:80), and this is an important process of cellulosome study by Metabolomics Group.
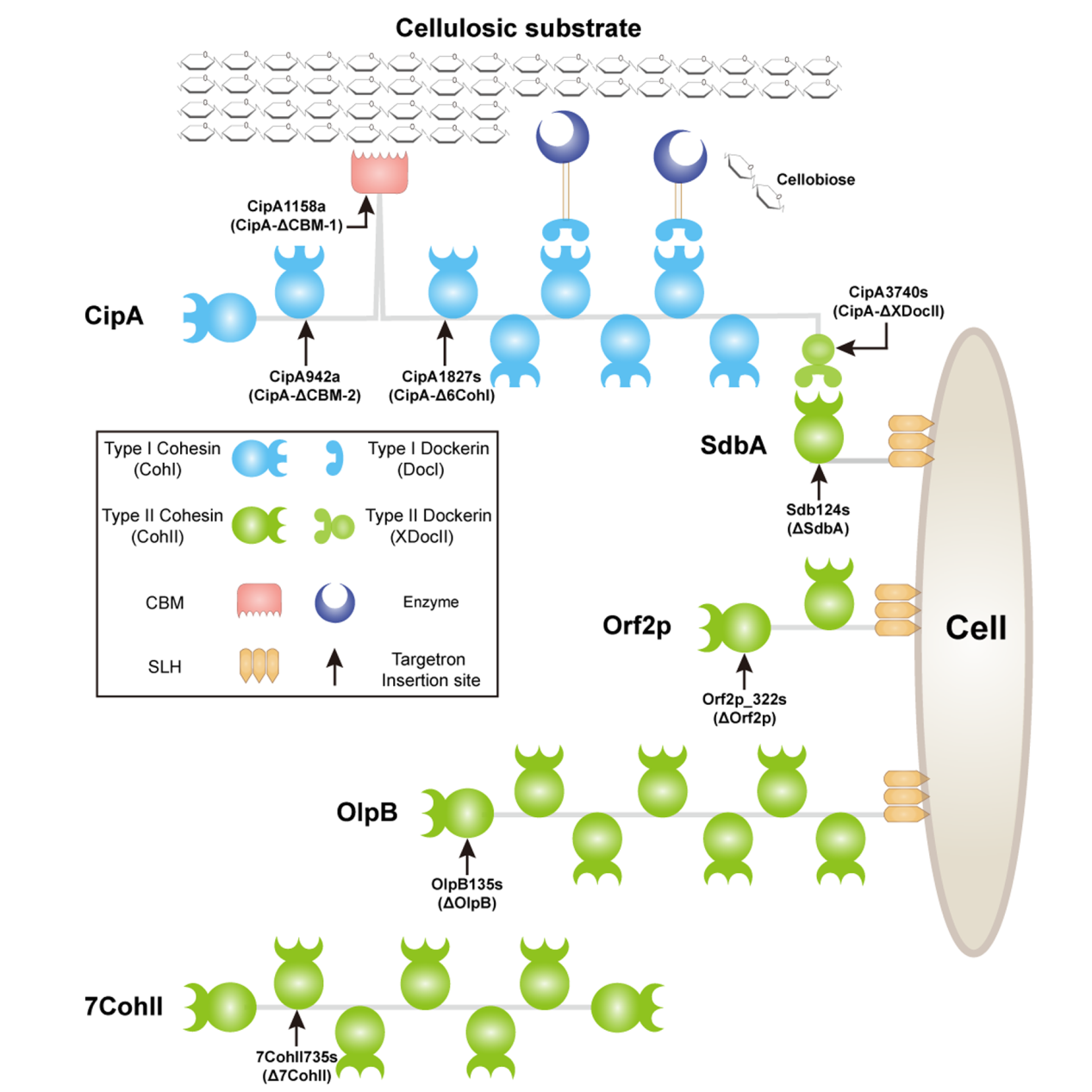
C. thermocellum is considered as a natural producer of lignocellulosic ethanol via consolidated bioprocessing route. This thermophilic anaerobic bacterium degrades cellulose efficiently using a highly effective cellulosome, a macromolecular complex consisting of multiple cellulose degrading enzymes and non-catalytic scaffoldins. The interactions between functional modules of scaffoldin proteins and enzymes result in the supermolecular structure of cellulosome, which provides synergy effects to guarantee the effective cellulose degradation. However, the contributions of various scaffoldins and synergy effects in cellulose degradation are still not clear.
To deeply understand the contributions of scaffoldins and their functional modules to cellulose hydrolysis by C. thermocellum, the research group constructed C. thermocellum mutants with truncated primary scaffoldin CipA or disrupted secondary scaffoldins, and analyzed cellulose hydrolysis, cellulosome formation, and cellulose binding of the mutants. The results indicated that the strong cellulosome-substrate synergy mediated by type I Cohesin modules and CBM is the core mechanics responsible for high efficient cellulose degradation, while the cellulosome-cell synergy mediated by type II Dockerin module showed relatively smaller contribution.
Compared with the primary scaffoldin, secondary scaffoldins showed moderately decreased contributions to cellulose hydrolysis, and their contributions are related to the number of type II Cohesin modules they have. Besides, the researchers found that the mutant lacking cell-associated polycellulosomes adheres to cellulose almost as strongly as wild-type cells, revealing an alternative, previously unknown cellulose-binding mechanism.
These findings illustrate the contributions of scaffoldins and the synergy effects to cellulose hydrolysis by C. thermocellum, provide new insights into cellulosome function mechanism, and impact genetic engineering of thermophiles for lignocellulose bioconversion. This study also demonstrates that the thermotargetron method can be wildly used in the genetic engineering in thermophiles with high efficiency, and will support the further research on thermophilic microorganisms and their industrial applications.
This research work is co-led by Professor CUI Qiu and associate professor LIU Yajun at QIBEBT. This study is supported by the National Basic Research Program of China (973 Program), the Instrument Developing Project of the Chinese Academy of Sciences, and the National Natural Science Foundation of China.
 |
|
Figure 1. Schematic representation of the analyzed primary scaffoldin CipA and secondary scaffoldins ofC. thermocellumDSM1313. (Image by Metabolomics Group, QIBEBT) |
Reference:
Contact:
Dr. LIU Ya-Jun, liuyj (AT) qibebt.ac.cn
Dr. CUI Qiu, cuiqiu (AT) qibebt.ac.cn
Web: http://english.qibebt.cas.cn