QIBEBT Developed a Series of Novel Metal-Organic Frameworks (MOFs) for Effective Energy Storage
Metal-Organic Frameworks (MOFs) are new types of crystalline porous materials constructed from the self-assembly of organic ligands and inorganic metal ions or metal clusters. Compared with conventional porous materials, MOFs have shown great potentials in the fields of energy gas (i.e. H2 or CH4) storage and CO2 capture due to their high surface area, tunable structural and amenable pore environments. It is urgently important to develop the MOFs with excellent performance and good stability for the utilization in energy storage applications.
The Hydrogen Storage & Novel Nano Materials Group at Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, headed by Professor ZHAO Xuebo, developed a series of novel MOFs for energy storage. To enhance the adsorption capacity for small molecular gases, a strategy of immobilizing Lewis Basic Bipyridinal sites into a Zr-based MOF through the self-assembly of a bipyridinyl ligand and Zr salts was employed. Thanks to the high surface area (2642 m2/g) combined with high-density of Lewis basic sites, this MOF shows high adsorption capacity for H2 (5.8 wt% at 77 K and 20 bar), CO2 (79.7 wt% at 293 K and 20 bar) and CH4 (180 v(STP)/v at 293 K, 35 bar). Moreover, this MOF exhibits ultrahigh thermal stability (the decomposing temperature is 512℃), and shows excellent stability when contacting common solvents and moisture. The excellent adsorption capacity combined with good stability allows this MOF to be a promising material for methane storage and carbon dioxide capture. This research result has been published on a recent Chemical Communication.
|
|
|
Figure 1. The structure of Zr-based MOFs with Lewis basic sites and corresponding adsorption isotherms (Image by Hydrogen Storage & Novel Nano Materials Group). |
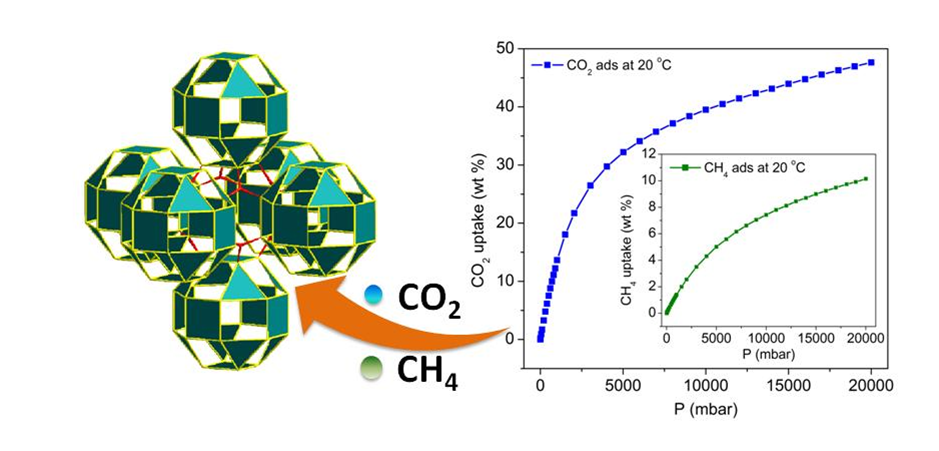
Fabricating sub-units such as novel Secondary Building Units (SBUs) or Supermolecular Building Blocks (SBBs) is an effective strategy for constructing novel MOFs. In this regard, the ligands containing isophthalate or iminodiacetic acid groups were used in this group to assembly with transitional metal ions (i.e. Zn2+, Co2+, Ni2+), leading to MOFs with novel metal-carboxylate nano-tube SBUs and small cubioctahedron SBBs. Notably, these MOFs show good performance in adsorption of CO2 and CH4. The corresponding results have been published on European Journal of Inorganic Chemistry and New Journal of Chemistry.
 |
|
Figure 2. The structural presentation of the MOF with metal-carboxylate nano-tube SBUs.(Image by Hydrogen Storage & Novel Nano Materials Group). |
 |
|
Figure 3. The presentation of MOF constructing from SBBs(Image by Hydrogen Storage & Novel Nano Materials Group). |
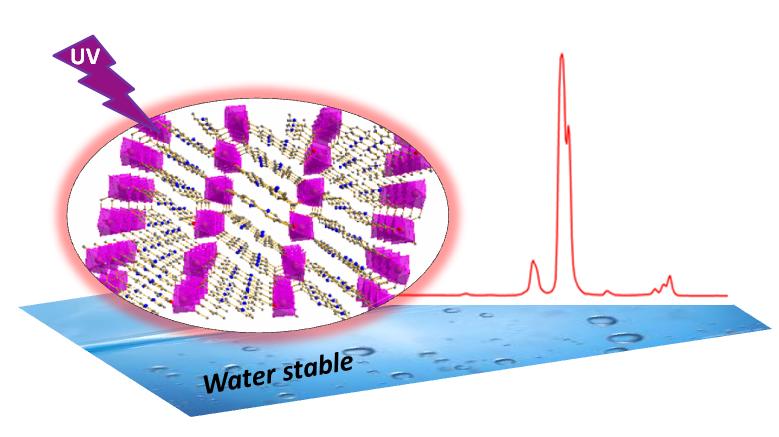
Based on a bipyridinyl carboxylate ligand and rare-earth metal ions (i.e. Y3+, Sm3+,Eu3+, Gd3+ etc), a series of isostructural MOFs with metal-craboxylate double chain SBUs were also constructed. Some of them show intensive luminescence at room temperature. This series of MOFs exhibit exceptional high thermal stability (decomposing temperature: 520~ 580 ℃) that is among the top ranks of MOFs with high thermal stability. Furthermore, these series of MOFs shows good water stability. Such advantages enable these MOFs to be very promising materials in practical applications. The corresponding results have been published on European Journal of Inorganic Chemistry.
 |
|
Figure 4. The structural presentation of a series of rare-earth metal MOFs and the luminescent spectra(Image by Hydrogen Storage & Novel Nano Materials Group). |
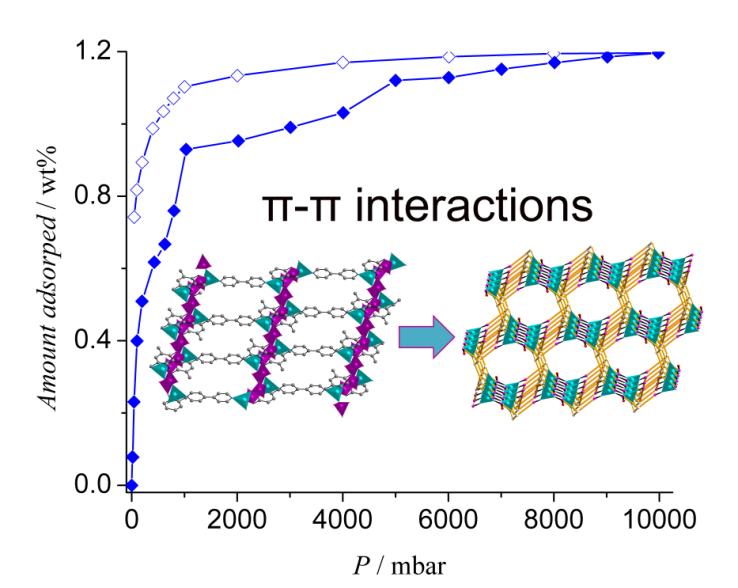
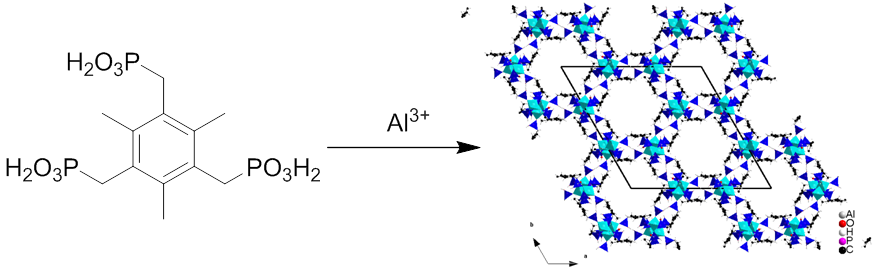
Other than the MOFs based on carboxylate ligands, the MOFs constructed from phosphonate ligand are another important branch in MOFs chemistry. The diverse coordination groups and coordination modes always lead to MOFs with unusual topologies and stabilities. On the other hand, the “breathing effects” and the self-recognition to guest molecules induced by network flexibility make such MOFs very attractive in the fields of molecular recognition, sensing and gas separation, etc. In Zhao’s group, some soft phosphonate ligands were synthesized and assembled with the Co2+, Ni2+ and Al3+, respectively, with the addition of 4,4’-bipyridine as auxiliary ligand, resulting in some novel phosphonate MOFs. Among them, a type of 3D phosphonate MOFs constructed from the orderly stacking of 2D layers through π-π interactions show breathing effects towards hydrogen and have very good water stability. A 3D phosphonate MOF with nano-scale hexagonal channels is the first case that has ever been reported. In addition, the starting materials used in the synthesis of this MOF such as ligands, metal salt and the solvent are low-cost and makes this series of MOF are of great potential for practical applications. These research results have been published on Chemistry-An Asian Journal and Dalton Transactions.
 |
|
Figure 5. The 3D phosphonate MOF constructed from the stacking of 2D layers throughπ-π interactions and the unusual H2isotherms.(Image by Hydrogen Storage & Novel Nano Materials Group) |
Figur 6. The 3D phosphonate MOF with nano-scale hexagonal channels constructed from a poly phosphonate ligand and Al3+(Image by Hydrogen Storage & Novel Nano Materials Group)
Contact:
Professor ZHAO Xuebo
Qingdao Institute of Bioenergy and Bioprocess Technology,
Chinese Academy of Sciences
Email: zhaoxb (AT) qibebt.ac.cn