OFET Materials Study: From Diazocines to Fused Heteroacenes Bearing Pyrrolo[3,2-b]pyrrole Core
As a potential building block for artificial muscle polymeric materials, diazocines are normally prepared from 2-aminobenzophenones by conventional condensation reaction, which requires long reflux time, and the yield varies dramatically with different substrates. Moreover, the preparation of 2-aminobenzophenones is fairly complicated and expensive, which limits the study of the performance of diazocines.
Prof. WAN Xiaobo’s research group, Biomass-based & Biomimic Polymers Group at Qingdao Institute of Bioenergy & Bioprocess Technology, Chinese Academy of Sciences, has made progress in the mechanism study of diazocine synthesis and the synthesis of novel organic field-effect transistor (OFET) materials derived from it.
WAN’s team developed a novel synthetic method for diazocines, with the cyclization of 2-benzoylbenzoyl azides under acidic conditions as the key step. This reaction is fast and efficient. This work has been published in Organic Letters (Org. Lett., 2011, 13, 709-711).
They further optimized the conditions and found that this reaction could occur under much milder conditions by using catalytic amount of acid at room temperature. They also clarified the reaction mechanism of which the details have been published in the latest issue of Tetrahedron (Tetrahedron, 2012, 68, 9665-9671).
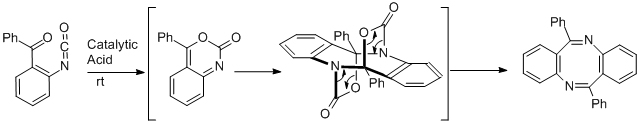 |
| Scheme 1. Optimized synthesis and revised mechanism of diazocines (Image by Prof. WAN Xiaobo) |
At the same time, WAN’s team expanded the potential application of diazocines, finding that novel fused heteroacenes bearing pyrrolo[3,2-b]pyrrole core could be successfully synthesized from 6,12-dichloro-diazocines via a novel reductive ring-closure methodology using zinc dust as the reducing reagent under acidic conditions. These compounds could be used as small molecular OFET materials and building blocks for OFET polymers. Being simple and efficient, this novel method is superior to the methodologies reported before for the synthesis of heteroacenes bearing pyrrolo[3,2-b]pyrrole core and could be extended to prepare larger conjugated systems.
With the cooperation with Prof. LIU Yunqi’s team at Institute of Chemistry, Chinese Academy of Sciences, the preliminary study on the OFET properties of these heteroacenes has been performed and the results indicated that these compounds were good potential OFET materials. This work has been published in the latest issue of Chemical Communication (DOI:10.1039/C2CC36689D).
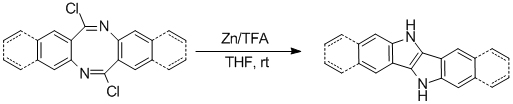 |
|
Scheme 2. One-step synthesis of fused heteroacenes bearing pyrrolo[3,2-b]pyrrole core from 6,12-dichloro-diazocines (Image by Prof. WAN Xiaobo) |